Triapine® (3-AP)
Ribonucleotide Reductase Inhibitor / Radiosensitizer
cGMP API - Type II DMF (US and Canada)
Oral Capsule - Phase 1 & 2 clinical studies (NCI and Northwestern)
The figure on the right shows the docked pose of Triapine®
coordinated to the diiron cluster of RNR2 (PDB ID: 1W68)
Ribonucleotide reductase (RNR) is a rate-limiting enzyme that mediates the production of deoxyribonucleotide triphosphates (dNTPs), the essential building blocks of DNA and is involved in DNA repair. RNR is composed of two non-identical regulatory subunits, with the second subunit (Regulatory subunit 2 [RRM2]) controlling RNR expression and activity. RRM2 contains a di-iron center that catalyzes the reduction of 2’ carbon of ribonucleotides and is critical to its function. RRM2 is a target of many signaling pathways frequently dysregulated in cancer such as KRAS, p53, and mTOR, and has been reported to be upregulated in several cancer types.
Triapine®, an iron chelator, is s synthetic heterocyclic carboxaldehyde thiosemicarbazone with potential antineoplastic activity being studied in the treatment of neuroendocrine tumors (NET) and glioblastoma multiforme (GBM). Triapine® inhibits the function of ribonucleotide reductase, specifically the RRM2 subunit. Also called 3-aminopyridine-2-carboxaldehyde thiosemicarbazone and 3-AP, Triapine® inhibits RNR, resulting in the inhibition of the conversion of ribonucleoside diphosphates to deoxyribonucleotides necessary for DNA synthesis and repair.
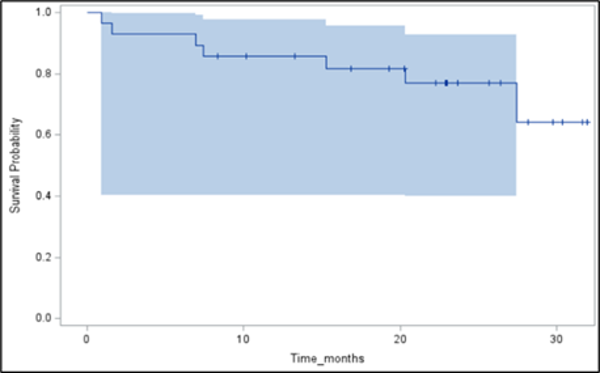
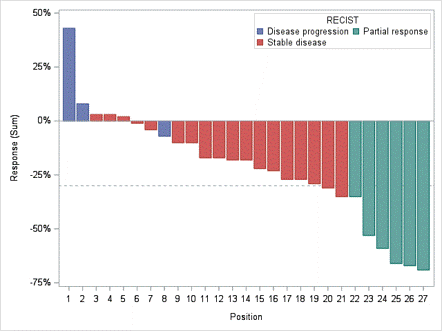
An oral presentation for the National Cancer Institute (NCI) sponsored Phase I Study 10388 (NCT04234568) “Testing the Addition of an Anti-cancer Drug, Triapine, to the Usual Radiation-Based Treatment (Lutetium Lu 177 Dotatate) for Neuroendocrine Tumors” at the European Society for Medical Oncology (ESMO) Congress was held on the October 20th Mini oral session: NETs and endocrine tumours “1709MO - Multi-center NCI-sponsored phase 1 study of triapine in combination with 177Lu-dotatate in patients with well-differentiated gastroenteropancreatic neuroendocrine tumours (GEP-NETs)” by Aman Chauhan, MD, Associate Professor of Medical Oncology, Leader of Neuroendocrine Tumor Program and Co-Director of Theranostics Program, University of Miami Sylvester Comprehensive Cancer Center. Among 28 patients evaluable for efficacy, the confirmed Objective Response Rate (ORR) of Triapine® with Lu 177 Dotatate was 21.4% (50% improvement compared to NETTER-1), and the median Progression Free Survival (PFS) was 38.0 months (improvement of 10 months compared to NETTER-1). The figure on the left shows the survival probability out to 28 months and the figure on the right shows the patient-specific NET size response in the study.
In the second dose cohort, median PFS has not yet been reached and exceeds 40 months (improvement of 12 months compared to NETTER-1). The NETTER-1 trial with Lu 177 Dotatate monotherapy established an ORR of 14% and a median PFS of 28.4 months. This promising treatment combination of Triapine® with Lutetium Lu 177 dotatate could reshape the treatment landscape for GEP-NETs and may offer a new therapeutic option for patients who have exhausted standard treatments. A 94 patient randomized phase 2 trial (NCT05724108) comparing the combination to standard 177Lu-Dotatate monotherapy is currently underway at 14 sites across the United States.
Clinical Studies (Active)
NCT06410248 Triapine in Combination With Temozolomide for the Treatment of Patients With Recurrent Glioblastoma
NCT04234568 Testing the Addition of an Anti-cancer Drug, Triapine, to the Usual Radiation-Based Treatment (Lutetium Lu 177 Dotatate) for Neuroendocrine Tumors
NCT06860594 Testing the Addition of an Anti-Cancer Drug, Triapine, to the Usual Radiation Therapy for Recurrent Glioblastoma or Astrocytoma
NCT02595879 Triapine With Chemotherapy and Radiation Therapy in Treating Patients With IB2-IVA Cervical or Vaginal Cancer
NCT05724108 Testing the Effectiveness of an Anti-cancer Drug, Triapine, When Used With Targeted Radiation-based Treatment (Lutetium Lu 177 Dotatate), Compared to Lutetium Lu 177 Dotatate Alone for Metastatic Neuroendocrine Tumors
NCT02466971 Testing the Addition of a New Anti-Cancer Drug, Triapine, to the Usual Chemotherapy Treatment (Cisplatin) During Radiation Therapy for Advanced-stage Cervical and Vaginal Cancers
NCT04494113 (Suspended) Testing the Response to the Anti-cancer Drug, Triapine, in Uterine Cancers Using Markers From the Tissue at the Time of Hysterectomy
Clinical Studies (Completed)
NCT00075504 Triapine and Gemcitabine Hydrochloride in Gallbladder Cancer
NCT00390052 3-AP in Treating Patients With Advanced or Metastatic Solid Tumors
NCT00335998 Phase I Study of Intravenous Triapine (IND # 68338) in Combination With Pelvic Radiation Therapy With or Without Weekly Intravenous Cisplatin Chemotherapy for Locally Advanced Cervical, Vaginal, or Pelvic Gynecologic Malignancies
NCT00293345 3-AP and Gemcitabine in Treating Patients With Advanced Solid Tumors or Lymphoma
NCT00085371 Triapine as First-Line or Second-Line Therapy in Treating Patients With Locally Advanced or Metastatic Cancer of the Pancreas
NCT00288093 3-AP and Radiation Therapy in Treating Patients With Stage III Pancreatic Cancer That Cannot Be Removed By Surgery
NCT00077350 A Phase II Trial of Triapine (NSC #663249) in Combination With Gemcitabine as Second Line Treatment of Non-Small Cell Lung Cancer
NCT00077181 3-AP and High-Dose Cytarabine in Treating Patients With Advanced Hematologic Malignancies
NCT00941070 Triapine, Cisplatin, and Radiation Therapy in Treating Patients With Cervical Cancer or Vaginal Cancer
NCT00077545 3-AP Plus Cisplatin in Treating Patients With Recurrent or Metastatic Adenocarcinoma of the Esophagus or Gastroesophageal Junction
NCT00095888 3-AP and Gemcitabine in Treating Patients With Refractory Metastatic Breast Cancer
NCT00081276 3-AP and Cisplatin in Treating Patients With Recurrent or Persistent Platinum-Resistant Ovarian Epithelial or Primary Peritoneal Cancer
NCT00079014 3-AP and Doxorubicin In Treating Patients With Metastatic or Refractory Solid Tumors
NCT00084877 Irinotecan and 3-AP in Treating Patients With Metastatic or Unresectable Solid Tumors
NCT00381550 3-AP and Fludarabine in Treating Patients With Myeloproliferative Disorders, Chronic Myelomonocytic Leukemia, or Accelerated Phase or Blastic Phase Chronic Myelogenous Leukemia
NCT00078975 3-AP and Gemcitabine in Treating Patients With Recurrent, Unresectable, or Metastatic Pancreatic Cancer
NCT00075660 3-AP in Treating Patients With Previously Untreated Locally Recurrent or Metastatic Renal Cell Carcinoma
NCT00006218 3-AP in Treating Patients With Advanced Cancer
NCT00054015 3-AP in Treating Patients With Advanced Prostate Cancer
NCT00064090 3-AP and Cytarabine in Treating Patients With Hematologic Cancer
NCT00064051 3-AP and Gemcitabine in Treating Patients With Unresectable or Metastatic Pancreatic Cancer
NCT00064064 3-AP and Gemcitabine in Treating Patients With Metastatic Non-Small Cell Lung Cancer
NCT00077558 3-AP Followed By Fludarabine In Treating Patients With Relapsed or Refractory Acute or Chronic Leukemia or High-Risk Myelodysplastic Syndrome
NCT00077415 3-AP and Gemcitabine as Second-Line Therapy in Treating Patients With Stage III or Stage IV Recurrent Non-Small Cell Lung Cancer
NCT00004213 3-Aminopyridine-2-carboxaldehyde Thiosemicarbazone in Treating Patients With Solid Tumors
NCT00016874 3-AP Plus Cisplatin and Paclitaxel in Treating Patients With Advanced or Metastatic Cancer
NCT00024323 Combination Chemotherapy in Treating Patients With Advanced Cancer
Intellectual Property
Nanopharmaceutics holds world-wide exclusive rights to U.S. patents 5,767,134, 5,869,676, 6,458,816 and related foreign filings. Two new composition-of-matter patents were filed in 2024 and 2025.
References (over 180 total)
Regulation of neurogenesis and neuronal migration by Rrm2 and Timp3 following seizures. Adeyeye AO, Varma P, Enerlan N, Coppin M, Hsieh J. Neurobiol Dis. 2025 Oct 15;215:107094. doi: 10.1016/j.nbd.2025.107094. Epub 2025 Sep 8. PMID: 40930429
The anticancer thiosemicarbazone triapine exerts immune-enhancing activities via immunogenic cell death induction and FAS upregulation. Stiller B, Stefanelli A, Schueffl H, Mathuber M, Skorokhyd N, Gufler J, Pirker C, Holcmann M, Panchuk R, Sibilia M, Marko D, Berger W, Kowol CR, Hager S, Heffeter P. Exp Hematol Oncol. 2025 Aug 22;14(1):109. doi: 10.1186/s40164-025-00700-0. PMID: 40847419
Disulfide-based 2-pyridyl-hydrazone prochelators induce iron deprivation and oxidative stress in ovarian cancer cells. Fussell JB, Shaw JP, Grams MA, Sung YS, Jheng RH, Astashkin AV, Tomat E. J Biol Inorg Chem. 2025 Aug;30(4-5):443-452. doi: 10.1007/s00775-025-02119-8. Epub 2025 Jul 1. PMID: 40591007
Incorporation of triapine (T) to cisplatin chemoradiation (CRT) for locally advanced cervical and vaginal cancer: Results from NRG-GY006, a phase III randomized trial. Leath CA 3rd, Deng W, Mell LK, Richardson DL, Walker JL, Holman LL, Lea JS, Amarnath SR, Santos-Reyes LJ, Arend RC, Mayadev J, Jegadeesh N, DiSilvestro P, Chon HS, Ghamande SA, Gao L, Albuquerque K, Chino JP, Donnelly E, Feddock JM, Lowenstein J, Quick AM, Kunos CM, MacKay H, Aghajanian C, Monk BJ. Gynecol Oncol. 2025 Mar 17;195:122-133. doi: 10.1016/j.ygyno.2025.03.007. PMID: 40101606.
Dose finding, bioavailability, and PK-PD of oral triapine with concurrent chemoradiation for locally advanced cervical cancer and vaginal cancer (ETCTN 9892). Taylor SE, Behr S, Cooper KL, Mahdi H, Fabian D, Gallion H, Ueland F, Vargo J, Orr B, Girda E, Courtney-Brooks M, Olawaiye AB, Randall LM, Richardson DL, Sullivan SA, Huang M, Christner SM, Beriwal S, Lin Y, Chauhan A, Chu E, Kohn EC, Kunos C, Ivy SP, Beumer JH. Cancer Chemother Pharmacol. 2024 Dec 14;95(1):4. doi: 10.1007/s00280-024-04720-1. PMID: 39673591 Clinical Trial.
Ribonucleotide reductase regulatory subunit M2 drives glioblastoma TMZ resistance through modulation of dNTP production. Ella N Perrault, Jack M Shireman, Eunus S Ali, Peiyu Lin, Isabelle Preddy, Cheol Park, Shreya Budhiraja, Shivani Baisiwala, Karan Dixit, C David James, Dieter H Heiland, Issam Ben-Sahra, Sebastian Pott, Anindita Basu, Jason Miska, Atique U Ahmed. Sci Adv. 2023 May 19;9(20):eade7236. doi: 10.1126/sciadv.ade7236. Epub 2023 May 17. PMID: 37196077.
Co-administration of Inulin and Iron Fortificants improves Iron Deficiency Biomarkers in Female Sprague Dawley Rats. Rizwan Ahmad AM, Farooq U, Anees M, Anis RA, Rashid S, Ahmed W. Food Sci Nutr. 2022 May 7;10(7):2141-2148. doi: 10.1002/fsn3.2337. eCollection 2022 Jul. PMID: 35844906.
Inhibitors of the Cancer Target Ribonucleotide Reductase, Past and Present. Huff SE, Winter JM, Dealwis CG. Biomolecules. 2022 Jun 10;12(6):815. doi: 10.3390/biom12060815. PMID: 35740940.
Replication stress induced by the ribonucleotide reductase inhibitor guanazole, triapine and gemcitabine in fission yeast. Alyahya MY, Khan S, Bhadra S, Samuel RE, Xu YJ. FEMS Yeast Res. 2022 Mar 24;22(1):foac014. doi: 10.1093/femsyr/foac014. PMID: 35262697.
Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Timoshnikov VA, Selyutina OY, Polyakov NE, Didichenko V, Kontoghiorghes GJ. Int J Mol Sci. 2022 Jan 23;23(3):1247. doi: 10.3390/ijms23031247. PMID: 35163169.